Drawing the Lewis Structure for BrF2
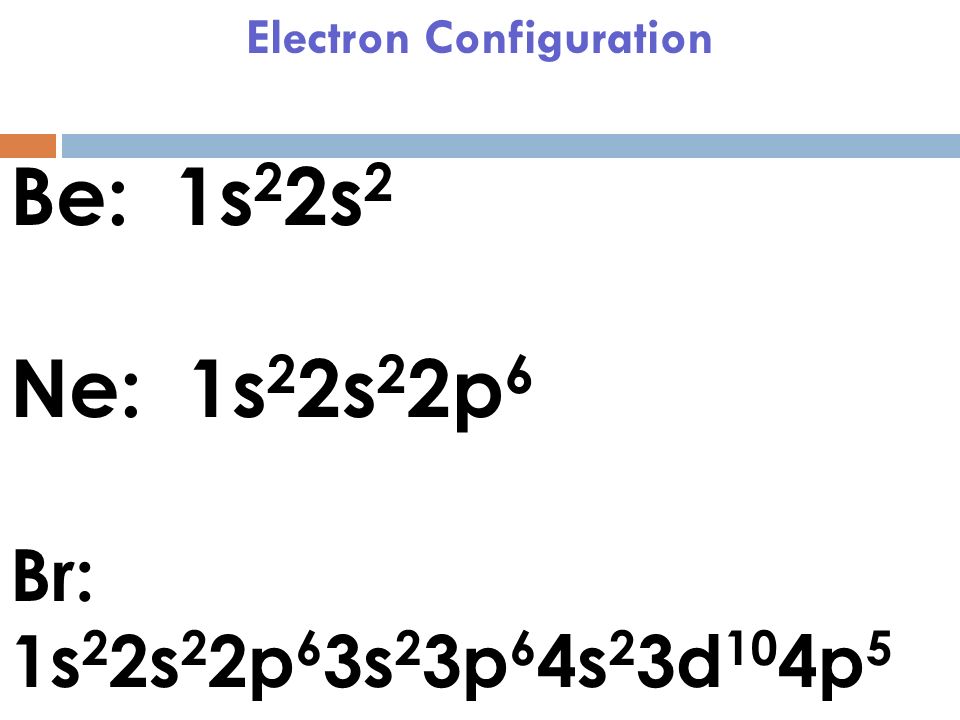
Answer: Bromine has 7 valence electrons. There are two ways to determine the number of valence electrons for bromine. One way is to write out the electron configuration for the element. This means that the central Bromine (Br) atom will have an odd number (9 total). Bromine is the least electronegative and goes at the center of the BrF 2 Lewis structure. Bromine can hold more than 8 valence electrons since it is in Period Four of the periodic table. For the BrF 2 Lewis structure there are a total of 21 valence electrons.
Viewing Notes:
Number Of Valence Electrons Chart
- With BrF2 there are an odd number of valence electrons (21 total). This means that the central Bromine (Br) atom will have an odd number (9 total).
- Bromine is the least electronegative and goes at the center of the BrF2 Lewis structure.
- Bromine can hold more than 8 valence electrons since it is in Period Four of the periodic table.
- For the BrF2 Lewis structure there are a total of 21 valence electrons.
- If you check the formal charges for BrF2 you will find that each atom has a formal charge of zero.
See the Big List of Lewis Structures

Valence Electrons Calculator

Transcript: This is the Lewis structure for BrF2. Bromine has 7 valence electrons, as does Fluorine. We have two Fluorines. So 7 plus 14 equals 21. So we have an odd number of valence electrons in the Lewis structure for BrF2. That means one electron is going to go unpaired. We'll put the Bromine in the center, it's the least electronegative, and we'll put a Fluorine on either side. We'll put a pair of electrons between the atoms to form chemical bonds. We've used 4. And then around the outside, 8, 10, 16, back on the center, 18, 20.
Valence Electrons In Brf3
So at this point in the Lewis structure for BrF2, we've used 20 valence electrons and we've completed the octets on each of the atoms. They all have 8. Bromine is in period 3 on the periodic table. That means it can hold more than eight valence electrons. So we have one valence electron left, and we're just going to put it right here on the Bromine. So we've used all the 21 valence electrons we started with, and the Fluorines have octets and the Bromine has nine valence electrons, but that's OK, it's in period 3. If you check the formal charges, you'll see that the formal charge for each atom is going to be zero.
So this is the Lewis structure for BrF2. This is Dr. B., thanks for watching.

